so42- lewis structure|Iba pa : iloilo A step-by-step explanation of how to draw the Sulfate Ion Lewis Dot Structure (SO42- ). We'll also look at the molecular geometry, bond angles, electron geometry, and resonance for the. 14M Followers, 3,064 Following, 667 Posts - Andrea Brillantes (@blythe) on Instagram: "Actress | Endorser | Content Creator | CEO & Founder of @luckybeautyinc | Certified Fan Girl "
PH0 · sulfation lewis formel
PH1 · nh4+ lewis structure
PH2 · ion sulfate schéma de lewis
PH3 · ion sulfate lewis
PH4 · Iba pa
Chicadulce69's Porn Videos And Images, Gifs, Leaks ., About Chicadulce69 I am a very outgoing, hot girl, I really like erotic and sexual content and get your attention, I just want to be sweet with youUnlock the potential of Deep Nude AI technology with Drawnudes.io! Effortlessly upload any photo to nudify and remove clothes, unveiling deep nude images. Explore the forefront of Undress AI and cloth remover capabilities, and experience the precision of Nude AI transformations. Dive into a world where technology meets creativity. Try it now for free .
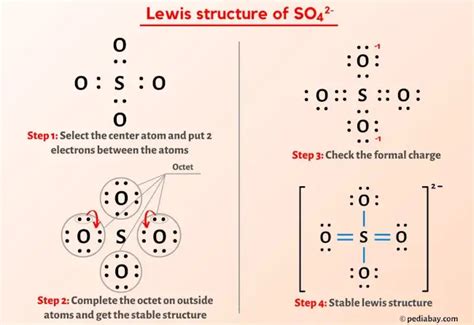
so42- lewis structure*******A step-by-step explanation of how to draw the SO4 2- Lewis Dot Structure (Sulfate ion). For the SO4 2- structure use the periodic table to find the total number of valence electrons .more. Learn how to draw the Lewis structure of sulfate ion (SO42-) with formal charge calculation and VSEPR theory. Find out the molecular geometry, hybridization, . SO42- Lewis Structure, Hybridization, Bond Angle and Molecular Geometry. SO42- is a chemical name for the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms, and a charge of -2. .
A step-by-step explanation of how to draw the Sulfate Ion Lewis Dot Structure (SO42- ). We'll also look at the molecular geometry, bond angles, electron geometry, and resonance for the.
This chemistry video explains how to draw the lewis structure of the sulfate ion SO4 2-.How To Draw Lewis Structures: https://www.youtube.com/wat.Lewis Structure for SO4 2- (Sulfate Ion) Commonly Tested Lewis Structures. We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and .The Lewis structure for SO 42- is requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis structure for SO 4 at first. .Learn how to draw the Lewis structure of SO4 2- (sulfate ion), a chemical species with intriguing properties. Follow the steps to determine valence electrons, choose the central .
Lewis structure of SO4 2- ion (Sulfate ion) contains two double bonds and two single bonds between the Sulfur (S) atom and Oxygen (O) atoms. The Sulfur atom .Drawing the Lewis Structure for SO 42- ( Sulfate Ion) Sulfates (salts with the SO 42-) are frequently used in industry and biologically. A commonly used sulfate is sodium lauryl ether sulfate found in shampoo, toothpaste, etc. For example, MgSO 4 is also known as Epsom Salts. There are 32 valence electrons available for the Lewis structure for .
Steps of drawing SO4 2- lewis structure Step 1: Find the total valence electrons in SO4 2- ion. In order to find the total valence electrons in SO4 2- ion, first of all you should know the valence electrons present in sulfur atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.) Hi Everyone! For today’s video, we are going to do SO42- Lewis Structure. It is a chemical formula for Sulfate ions. To determine its Lewis Structure, we fir. The SO42- Lewis structure depicts the molecular arrangement of sulfate, which consists of one sulfur atom and four oxygen atoms. The structure has two double bonds and two single bonds arranged around the sulfur atom, with each of the four oxygen atoms attached to it. Within this arrangement, the oxygen atoms that form double bonds .Lewis Dot of the Sulfate Ion. SO 42-. Back. 70 More Lewis Dot Structures. S does not follow the octet rule. It will hold more than 8 electrons. Sulfur having valence electrons in the 3rd energy level, will also have access to the 3d sublevel, thus allowing for more than 8 electrons. Elements in the first 2 periods of the Periodic Table do not . To determine the number of lone pairs and bonding pairs of electrons for SO4 2- we first need to draw as valid Lewis Structure. Once we have a Lewis Structur.so42- lewis structure There are equivalent six resonance structures SO4 2- the Sulfate ion. We start with a valid Lewis structure and then follow these general rules.- Resonance .Iba pa The Lewis structure of a sulfate [SO4]2- ion consists of 1 sulfur (S) atom and 4 atoms of oxygen (O). The sulfur atom is present at the center of the Lewis structure while the oxygen atoms occupy terminal positions. There are a total of 4 electron density regions around the central S atom in the Lewis structure of [SO4]2-.Estructura de Lewis del [SO4]-2 (Ion Sulfato) Anuncios Adsense. Marcar. Estructura de Lewis de la Sacarosa (C12H22O11) Estructura de Lewis del NO3- (Ion Nitrato)The Lewis Dot Structure for SO 4 2-: The sulfate anion (SO 4 2-) results from the complete ionization of sulfuric acid (H 2 SO 4 ). The representation of the valence electron distribution is properly shown by drawing the Lewis structure.Total valence electrons concept is used to draw the lewis structure of SO 4 2-. In lewis structure of sulfate ion, there should be charges on several atoms due to -2 charge. Sulfate ion | sulfate ion | SO 4 2-Sulfate ion is one of the oxyanion of sulfur. Sulfur is at +6 oxidation state in SO 4 2-. Also, sulfate ion has a -2 charge. Lewis . Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, . The final Lewis structure of SO shows sulfur bonded to oxygen with a single bond, . (SO4^2-). To determine the Lewis structure, we start by counting the total number of valence electrons in the ion. In this case, sulfur contributes 6 valence electrons, while each oxygen atom contributes 6 valence electrons as well. . Chemistry learning made easy.This tutorial will help you deal with the lewis structure and moleculargeometry of sulfate ion (SO4 2-)
A quick explanation of the molecular geometry of SO4 2- including a description of the SO4 2- bond angles.Looking at the SO4 2- Lewis structure we can see th.
The steps to draw the Lewis structure of SO4 2- are as follows: 1. Determine the total number of valence electrons in the molecule. In this case, sulfur (S) has 6 valence electrons and each oxygen (O) atom has 6 valence electrons. Since there are four oxygen atoms, the total number of valence electrons is 6 + (6 * 4) + 2 = 32.In the Lewis dot structure for Nitrate ion Nitrogen atom is the least electronegative atom and goes at the center of the structure surrounded by two oxygen atoms. Placing one electron pair to show the chemical bond between each Nitrogen and Oxygen. Now we are left with 14 valence electron.
The Lewis structure of SO42- contains two single bonds and two double bonds, with sulfur in the center, and four oxygens on either side. The top oxygen atom and bottom oxygen atom has three lone pairs, the left oxygen atom and right oxygen atom has two lone pairs, and the sulfur atom does not have any lone pair.
15,036 filipina lesbian FREE videos found on XVIDEOS for this search.
so42- lewis structure|Iba pa